To be GREEN
does not mean that You must give up everything in Your everyday life
like eating steaks and selling Your car or moving to a forest and joining some tribe.
To be GREEN
means to take responsibility and to take small steps to minimize CO2 impact
and to preserve the nature.
This is where the renewable energy
comes in and this is exactly what we work with.
Our mission is to provide products and services to consumers that are based on renewable energy, specifically on green hydrogen
.
How is the green hydrogen
produced?
There are many different ways for producing green hydrogen
.
Here are some of the methods that we use and how they work:
Proton Exchange Membrane Electrolyzer
A PEM splits water into its constituents of oxygen and hydrogen employing acidic
electrolytes. In the acidic electrolytes, the positive ions are transported that are
known as cations. In water electrolysis, the protons are transferred from anode to
cathode in the ionic transport. The overall electrochemical reactions are listed as
follows:
Anodic reaction:
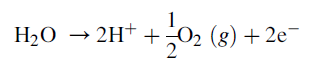
Cathodic reaction:
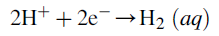
The operating temperature of the PEM electrolyzer is ranged 25-80°C. The characteristic
consumption of electricity for a commercial PEM electrolyzer is ranged
from 540 to 580 MJ/kg or 23-26 MJ/Nm3 of hydrogen production. The PEM electrolyzer
energy efficiency drops with current density quasi-exponentially and approaches
to 54% at 10 kA/m2 undergoing 80%-85% conversion and produced hydrogen is
more than 99.999% pure. The industrial PEM electrolyzer capacity ranged from 0.2
to 60 Nm3/h or 0.01 to 2.5 kg/h. The electricity consumption of a commercial PEM
electrolyzer can reach 400 kW with water consumption of approximately 25 L/h.
The following figure depicts the general illustration of a proton exchange membrane electrolyzer.
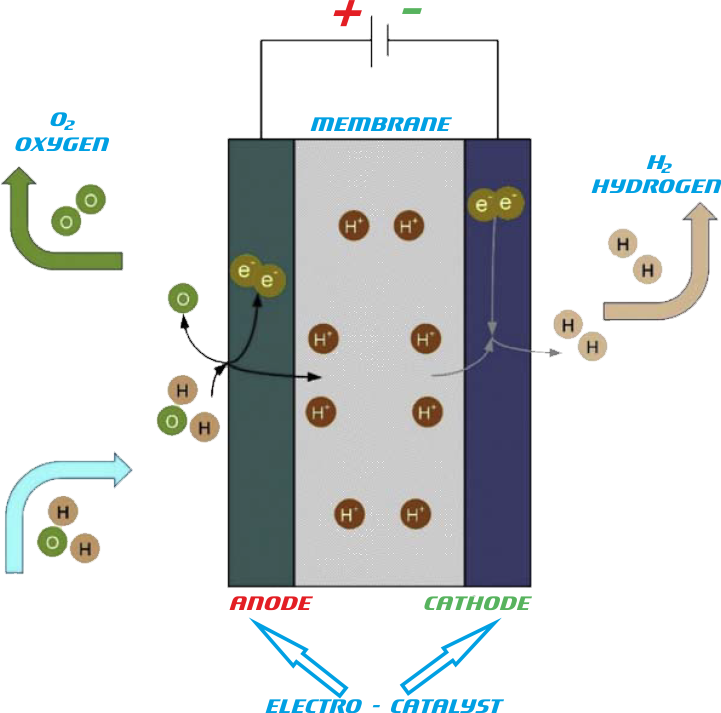
It includes both-sided channeled bipolar plates with two endplates. Each plate is attached to a porous, planar, and electrically conductive layer serving as anode or cathode, and a proton exchange membrane is positioned between electrodes. A polytetrafluoroethylene is more suitable than polystyrene sulfonate polymer for electrolyte as it offers better conductivity. Water flow management in PEM electrolyzer is a significant design subject as protons form hydronium ions and for that reason, membranes need to be hydrated to operate. For the reduced ohmic losses and better performance, the membrane-electrode assembly is given significant importance in the PEM electrolyzer design. Noble metal such as platinum catalysts can be coated on the electrodes to recompense for slow kinetics that is designed in a three-phase boundary. The electrical potential gradient is the most substantial driving force used to transport charge in the membrane cell process that is formed due to anions depletion on anode surface while cations depletion takes place on the cathode surface. Consequently, the cations are transported from anode to cathode partition. The conductivity shows the capability of a material to conduct electric current. To relate the resistance of a conductor with conductivity, the following correlation is used:
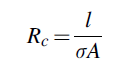
where Rc signifies the conductor resistance, s denotes the conductivity, A indicates area, and l represents the conductor length. Ohmic losses calculations use the specific membrane electrical conductivity equations that is determined using the following correlation presented by Ni el al.66:
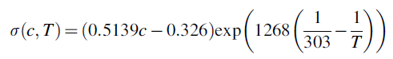
Here, T signifies membrane temperature, and c denotes water molar concentration inside the membrane. Molar concentration changes across membrane thickness from high anodic concentration to lower cathodic concentration. The assumption of the linear change in molar water content, across the membrane is reasonable.
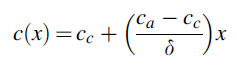
The electrical conductivity is well defined as
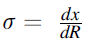
where R represents electrical resistance and differential equation can be represented as
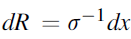
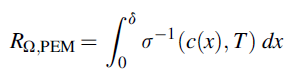
The PEM electrolyzer concentration overpotentials and water vapor concentration variation across the membrane are essential to be taken into consideration. The correlation for the concentration overpotential can be expressed as follows:
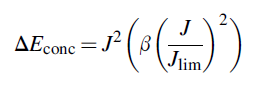
The factor ß can be defined as follows:
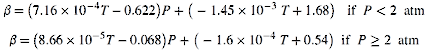
where
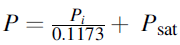
is local cathodic or anodic pressure, Pi denotes cathodic or anodic partial pressure, and Psat signifies water saturation pressure and index i is denoted as c for cathode and a for the anode. The cathodic and anodic activation potential in the PEM electrolyzer undergoes electron transfer at the membrane-electrode assembly. Ni et al.66 research article helps to determine the correlation for activation potential as follows:
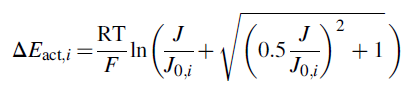
where J0,i is cathodic and anodic exchange current density. Exchange current density is a substantial constraint that helps to calculate activation overpotential. The capability of the electrode is symbolized in the electrochemical reaction. High exchange current density indicates high electrode reactivity that outcomes lower overpotential. The Arrhenius equation can be used to express electrolysis exchange current density:
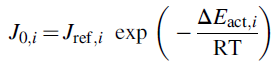
where Jref,i represents preexponential factor.
As shown above it requires power to produce H2 through electrolysis.
If we used the conventional powersource we would still have a CO2 impact as most of the conventional energy is generated using fossile fuels.
This is why WE only use renewable energy sources for H2 production and this is why hydrogen produced by us is GREEN HYDROGEN
We use Solar power, hydro power and wind power
for production of green H2.
Here is an example of our solar H2 production cycle:
Solar energy is recognized as a primary renewable energy source that can be
employed for producing clean and sustainable hydrogen. This solar energy source
has the potential to play a vital role in the transition from conventional to renewable
energy sources.
A recent study71 conducted the energetic, exergetic, and economic assessment of a
solar energy-driven hydrogen production system. The designed system consisted of
photovoltaic panels, proton exchange membrane (PEM) electrolyzer, PEM fuel cell,
and hydrogen storage unit, and the proposed system was investigated using a potential
software package TRNSYS. The net photovoltaic (PV) panel area was 300 m2 integrated
with the fuel cell of 5 kW capacity, and hydrogen is compressed at 55 bars
pressure for storage. The significant objective of this research study was to validate
that system covered emergency electricity demand without undergoing a shortage.
The analysis was performed for the whole year, and exergetic and energetic efficiencies
were found to be 4.25% and 4.06%.
The consumption of solar energy has been increasing significantly around the
globe due to the reduced cost, flexible products and incentives for applications.
Continuous and significant growth of solar energy sources can be depicted in all regions
of the world including North America, South and Central America, Europe, Commonwealth Independent States,
Middle East, Africa, and the Asia Pacific from 2008 to 2018. Solar energy consumption in North
America increased from 805 to 57,118 MW in the years 2008-18, increased from
39 to 7206 MW in South and Central America, increased from 10,522 to
128,758 MW in Europe, increased from 0 to 600 MW in the Commonwealth Independent
States, increased from 10 to 3181 MW in the Middle East, increased from
65 to 6093 MW in Africa and increased from 2955 to 284,873 MW in the Asia Pacific
region.
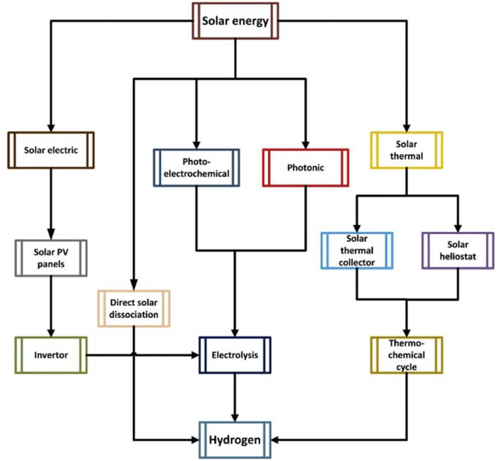
Following figure exhibits the routes of solar energybased hydrogen production.
A recent study72 was directed to the hydrogen production using renewable energy.
Water electrolysis is a typical hydrogen production commercial technology that is converted
to the renewable energy-based system if integrated with solar and wind energy
sources. The biomass processes carry the potential of producing hydrogen
using municipal sewage and forest residue employing the processes of gasification,
fermentation, and pyrolysis.
Hydrogen can be employed as an efficient and clean source in various applications,
namely fuel cells, transportation, portable applications, power, combustion, and
heating. Hydrogen can be produced through environmentally benign resources using
renewable sources such as wind, biomass, solar, geothermal and hydropower.
Furthermore, hydrogen helps to utilize the intermittent nature of renewable energy sources,
namely solar and wind.